This post will cover what will be tested during the Mid Year examination, for chemistry.
Basically, it will be mainly tested on acid and alkali.
So, everything will be here, and be prepared to take down notes, if you dont want to refer to your own notes.. =X
Defination of
acid: A substance that produces hydrogen ions (H+) as the only positive ions when it is dissolved in water
Physical Characteristics of
acid:
--- pH <7>Defination of
alkali: A substance that produces hydroxide ions (OH-) as the only negative ions when it is dissolved in water
Physical Characteristics of
alkali:
--- pH >7
--- Bitter taste
--- Caustic (Burning sensation, only for very concentrated ones)
--- Soapy feeling
--- Turns red litmus paper blue
--- Conducts electricity due to presence of mobile ions
Testing for various gases:
----
Ammonia (NH3): Test with damp/moist red litmus paper
-- Observation: Colourless, pungent gas turns damp red litmus paper blue
----
Carbon Dioxide (CO2): Test by bubbling gas into limewater (Calcium hydroxide)
-- Observation: Colourless, odourless gas forms a white precipitate with limewater (Calcium hydroxide)
----
Chlorine (Cl2): Test with damp/moist blue litmus paper
-- Observation: Yellowish, pungent gas turns damp blue litmus paper red and then bleaches
----
Hydrogen (H2): Test by inserting a lighted/burning splint into the test tube
-- Observation: Splint extinguishes with a "Pop" sound
----
Oxygen (O2): Test by inserting a glowing splint into the test tube
-- Observation: Colourless, odourless gas relights a glowing splint
----
Sulfer Dioxide (SO2): Test by bubbling gas into - Aqueous potassium dichromate (VI) or Acidified potassium manganate (VII)
-- Observation: Colourless, pungent gas turns - 1. Aqueous potassium dichromate (VI) from orange to green /or/ 2. Acidified potassium manganate (VII) from purple to colourless
Reaction of acids with metal carbonates:
#001: Effervescence occurs.
#002: Carbon Dioxide gas is produced.
Word Equation: Acid + Carbonate = Salt + Water + Carbon Dioxide
Example: Hydrochloric acid + Zinc carbonate = Zinc chloride + Water + Carbon Dioxide
Reaction of acids with metals:
#001: Effervescence occurs.
#002: Hydrogen gas is produced.
Word Equation: Acid + Metal = Salt + Hydrogen
Example: Nitric Acid + Iron = Iron Nitrate + Hydrogen
Reaction of acids with alkali:
#001: No effervescence occurs.
#002: Nothing is produced.
Word Equation: Acid + Alkali = Salt + Water
Example: Sulfuric Acid + Calcium Hydroxide = Calcium Sulfate + Water
Good Natural Indicators: Red Cabbage & Beetroot
Indicators:
- Methyl Orange
--- pH range: Below 3.1 = Red, Above 4.4 = Yellow
--- Turns Acid to Red, and Alkali to Yellow
--- Chemical name: p-dimethylamino-azobenzensulfonic acid, C14H35N3O3S
--- Suitable to be used for acidic solution
- pH paper
--- pH range: 1 to 14
--- Usually used to test pH value of soils and urine
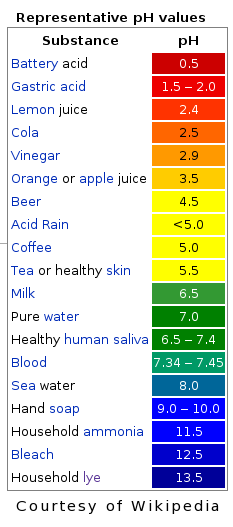
- Universal Indicator
--- Original colour: Dark Green (Very concentrated)
--- Turns Acid to red, orange or yellow/turns Alkali to blue or purple (Based on the level of concentration)
- Phenolphthalein
--- Original colour: Colourless
--- Cannot be used to distinguish acid from water
--- Turns Alkali to Pink, no colour change in Acid
--- In solutions containing pH below zero, phenolpthalein turns right orange in colour
--- In solutions that are very alkaline or basic, phenolphthalein turns purple in colour
If you have any queries or doubts, feel free to sms or email me at merlion0512@hotmail.com
And thankyou for visiting!
All the best for your examinations, and please visit my personal blog even if you do not need this blog anymore: http://her--weishi-shewii.blogspot.com/
Weishi!
Labels: Science
